G-18, SnehKunj Colony, Vyara, Dist. Surat, Gujarat – 394650
CALOHIE CG – TripleSynergy Antibiotic Adjuvant
(Ceftriaxone/Sulbactam/Disodium EDTA) {CARBAPENEM SPARING-OPTION}
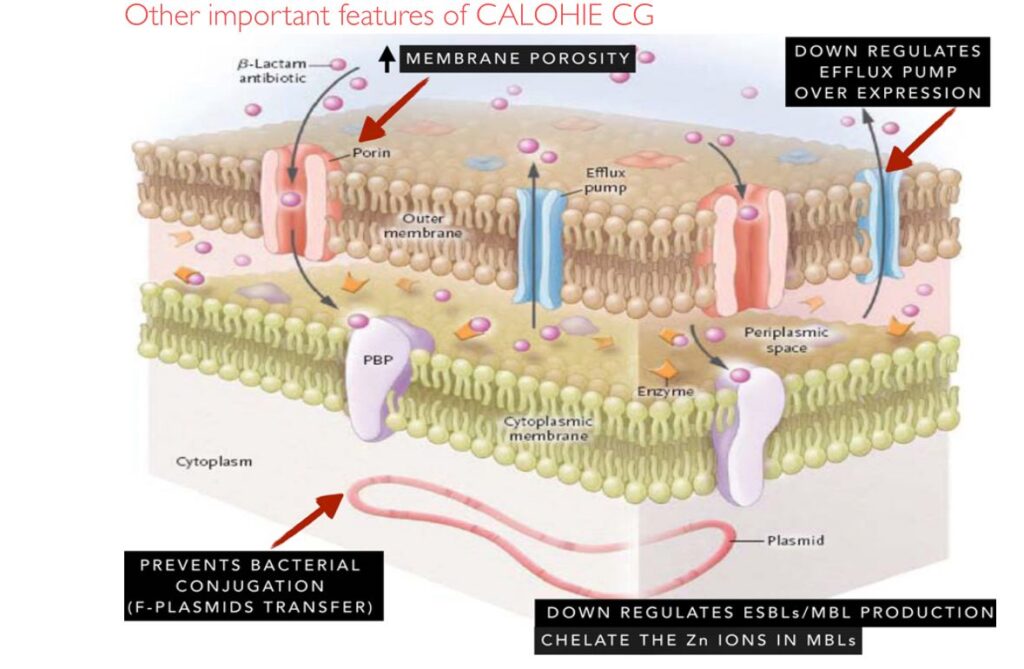
Mechanism of Action: Restoring Ceftriaxone Activity
- Ceftriaxone (3rd-gen ceph): Disrupts cell-wall synthesis in Gram-negatives. Its spectrum is normally compromised by β-lactamases.
- Sulbactam (β-lactamase inhibitor): Irreversibly inactivates serine β-lactamases (ESBLs, AmpC). By “sacrificing” itself, it protects ceftriaxone from hydrolysis, extending activity against ESBL-producing strains.
- Disodium EDTA (metal chelator): Uniquely targets metallo-β-lactamases (MBLs) by chelating their Zn²⁺ cofactor. This restores carbapenemase producers’ susceptibility; e.g. Ca-EDTA markedly lowered imipenem MICs in IMP/VIM-producing P. aeruginosa. EDTA also permeabilizes Gram-negative outer membranes (chelating Ca/Mg in LPS), boosting ceftriaxone uptake.
- Efflux inhibition: EDTA down-regulates efflux pumps (e.g. AcrAB-TolC). In one study, adding EDTA (10 mM) to ceftriaxone/sulbactam (CSE) produced a 32-fold MIC drop in efflux-positive E. coli (vs 8-fold for meropenem). This indicates EDTA inhibits efflux and increases intracellular drug levels.
- Biofilm dispersal: EDTA chelates ions in biofilm matrix, collapsing the structure. It has been shown to eradicate mature Gram-negative biofilms and markedly improve antibiotic kill.
- Plasmid “curing”: Classic studies found EDTA alone eliminated R-plasmids in E. coli, ablating antibiotic resistance markers. This conjugation-blocking effect helps prevent spread of resistance genes.
Callout: CSE (ceftriaxone+sulbactam+EDTA) is “a novel combination…with activity against multidrug-resistant Gram-negative pathogens.” Each component acts on different resistance mechanisms, restoring ceftriaxone efficacy against ESBL/MBL producers.
Pathogen Targets & Spectrum of Activity
- Enterobacterales: E. coli, Klebsiella spp., Enterobacter, Proteus, Serratia, etc., including ESBL-producers. In ICU surveillance, CSE was 94% active against carbapenem-sensitive Enterobacteriaceae.
- Non-fermenters: Pseudomonas aeruginosa, Acinetobacter baumannii, including carbapenem-resistant strains. CSE showed ~97% susceptibility against carbapenem-resistant Acinetobacter/Pseudomonas in one study.
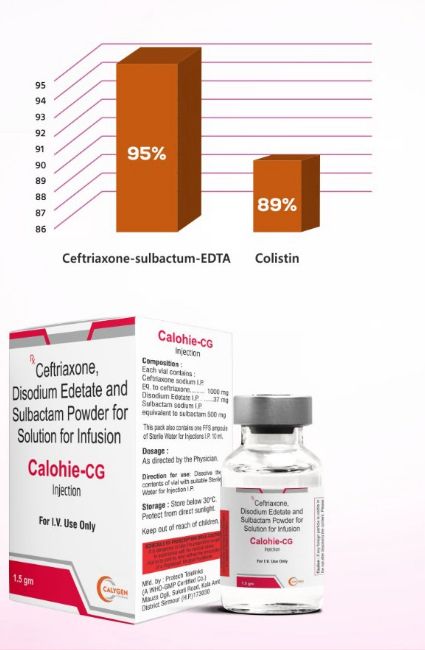
- Resistance phenotypes: Specifically potent against ESBL- and MBL-producing Gram-negatives. In a large collection of ESBL/MBL isolates, CSE inhibited 95% of strains, outperforming colistin (95% vs 89%).
- Critical-priority pathogens: Effective against WHO “Priority 1” MDR bugs. For example, in one ICU sample (n≈1877), E. coli/Klebsiella (56% of isolates), Acinetobacter (23%), and Pseudomonas (15%) dominated – all showing high susceptibility to CSE.
- Clinical infections: Indicated for moderate-to-severe infections where resistant Gram-negatives are suspected: complicated UTI/AP, hospital/VAP pneumonia, complicated skin/soft-tissue infections, osteomyelitis, sepsis, etc. By addressing both ESBLs and MBLs, CALOHIE CG can replace carbapenems in many such cases.
Clinical Evidence: Trials & Outcomes
- Phase III cUTI vs Ceftriaxone: In a randomized trial (n≈204), CSE-1034 (2g/1g/74mg q24h) outperformed ceftriaxone alone in complicated UTI. Composite cure (clinical + microbiological) was 97% (68/70) for CSE-1034 vs 83% (58/71) for ceftriaxone (Δ = +12.6%; 95% CI 5.9–26.4%). Adverse events were fewer with CSE-1034 (21% vs 36%).
- Phase III cUTI vs Meropenem (PLEA trial): In a double-blind trial (n=230), CSE-1034 was noninferior to meropenem for cUTI/AP. Symptomatic cure at test-of-cure was 95.9% vs 89.9%; eradication+symptom cure was 94.6% vs 87.0%. Microbiologic cure was 94.6% (CSE) vs 88.4% (meropenem). Authors conclude: “CSE…support[s] use…as a carbapenem-sparing treatment for patients with cUTI/AP caused by resistant Gram-negative pathogens.”.
- Phase III Superiority (cUTI): The CSE-1034 vs ceftriaxone study also demonstrated superiority. At test-of-cure, CSE-1034’s 97% cure rate (vs 83%) showed statistically significant benefit, establishing efficacy and a favorable bacteriologic response.
- Post-Marketing Surveillance: In 2,500+ real-world MDR infection cases, CSE-1034 achieved 79.4% cure and 20% improvement; only 0.2% failed. Common AEs (8.4% of patients) were mild (vomiting 3.0%, injection-site pain 2.5%, nausea 2.3%, rash/redness ~2%). No grade-4/5 toxicities were reported.
- PK/PD Profile: Adults received 1.5 g CSE q12h (3 g/day). At this regimen, EDTA C_max ≈25 mg/L, sufficient for MBL inhibition. Ceftriaxone’s long half-life and high protein binding allow once- or twice-daily dosing. Pharmacodynamic targets (fT>MIC) are met for typical Gram-negative MICs, as supported by the high clinical cure rates.
Clinical Highlight: In complex UTI, CALOHIE CG (2 g/1 g/74 mg) achieved 97% cure vs 83% with ceftriaxone alone (p=0.01). It also matched meropenem’s efficacy (94.6% vs 88.4% eradication), validating its role as a carbapenem-sparing agent.
Unique Advantages of Disodium EDTA
- MBL Enzyme Inhibitor: EDTA’s metal chelation is unique among β-lactam adjuncts. It directly inhibits NDM/VIM/IMP enzymes (IC₅₀ ≈55 µM for IMP-1). In animal pneumonia models, Ca-EDTA+imipenem dramatically reduced MBL-Pseudomonas lung counts versus antibiotic alone.
- Membrane Permeabilizer: By stripping divalent cations from lipopolysaccharide, EDTA increases outer membrane permeability. This “opens the door” for ceftriaxone and sulbactam to reach periplasmic targets in highly resistant bacteria.
- Anti-Biofilm: EDTA disrupts biofilm matrices. Studies show that combining EDTA with antibiotics can eradicate dense Gram-negative biofilms (preventing adherence and breaking existing biofilm). This enhances killing of persistent infections (e.g. catheter-related).
- Efflux Inhibition: EDTA impairs energy-dependent efflux. In vitro, EDTA downregulated AcrAB-TolC pump genes 2–2.4-fold (at 10 mM). The net effect: synergistic MIC reductions (e.g. 32-fold for CSE vs 8-fold for meropenem in one study).
- Plasmid/Curing Effect: Historical data show EDTA can eliminate resistance plasmids. In one classic study, EDTA-Tris alone “eliminated an antibiotic resistance marker” in an E. coli strain. This suggests EDTA may help block horizontal gene transfer of resistance.
- Safety Profile & Drug Interactions
- Adverse Events: CALOHIE CG’s safety mirrors its components. In trials, adverse events were mostly mild-to-moderate. Common AEs included GI upset (nausea, vomiting), injection-site reactions, rash and fever; all reported events were grade 1–2. No severe (grade ≥3) or unexpected toxicities occurred. Compared to ceftriaxone alone, CSE had a lower AE rate in one trial (21% vs 36%).
- Organ Systems: Hematologic: Rare neutropenia or thrombocytopenia (cephalosporin-class effect) may occur with prolonged use. Monitor CBC in long courses.
- Hepatobiliary: Ceftriaxone can cause biliary sludging or cholestasis; monitor LFTs if therapy exceeds 7–14 days. Avoid in neonates (risk of bilirubin displacement).
- Renal: Sulbactam and EDTA are renally excreted. Adjust dose if creatinine clearance <30 mL/min. Ensure adequate hydration.
- Electrolytes: EDTA chelates Ca²⁺/Mg²⁺. Avoid IV calcium during infusion (risk of chelate formation and hypocalcemia). No routine electrolyte binding is expected at the EDTA dose used (37 mg q12h), but prolonged use in critical patients warrants monitoring.
- Drug Interactions: No major CYP drug-drug interactions. Interaction profile is as per β-lactams:
- Calcium: Do not co-administer IV calcium products with CALOHIE CG (risk of ceftriaxone-calcium precipitates). Stagger infusions by ≥48 hours if necessary.
- Anticoagulants: Cephalosporins (including ceftriaxone) may enhance warfarin/heparin effect (inhibit vitamin-K–dependent clotting). Monitor coagulation parameters.
- Aminoglycosides: Concurrent use may increase nephrotoxicity risk. Monitor renal function if used together.
- Contraceptives: Antibiotics can alter gut flora; advise additional contraception during therapy.
- Others: No formal interactions reported for EDTA.
Special Populations
- Pregnancy (Category B): Ceftriaxone and sulbactam have no proven fetal risk in animal studies and are widely used when needed. EDTA’s reproductive safety data are limited, but given its low dose and lack of direct toxicity, CALOHIE CG can be used in pregnancy if the infection risk justifies it. (As always, reserve in first trimester unless essential.)
- Breastfeeding: Both ceftriaxone and sulbactam are excreted in human milk (sulbactam ~0.5 µg/mL). These levels are low, but infant exposure can occur. Monitor breastfed infants for diarrhea or thrush; avoid breastfeeding 24 h after dose if serious risk is present. EDTA is not known to concentrate in milk.
- Geriatric: No age-specific contraindications; however, elderly patients often have reduced renal function. Adjust dosing if creatinine clearance <30 mL/min (sulbactam/EDTA accumulate). The clinical trials included geriatric patients with similar efficacy and safety profiles.
- Pediatrics: Pediatric dosing (age >1 month) is 30–75 mg/kg q12h (max ~1.5 g q12h). Neonates (<1 month) should not receive ceftriaxone (risk of bilirubin displacement). Use only under specialist guidance.
Resistance-Breaking Supportive Studies
- Efflux: In E. coli, EDTA in CSE dramatically reduced AcrAB-TolC efflux pump expression and MICs. This highlights EDTA’s ability to reverse active drug expulsion.
- Biofilms: EDTA-containing combinations have been shown to eradicate mixed Gram-negative biofilms in vitro, supporting its use in device-associated infections (e.g. catheters).
- Conjugation: EDTA’s plasmid-curing effect (e.g. eliminating R-plasmids) suggests long-term stewardship benefit: reducing spread of ESBL/MBL genes in the patient’s flora.
Positioning & Launch (Calygen)
Calygen Pharmaceuticals will be first to market with CALOHIE CG post-patent expiry, filling a critical gap in antimicrobial therapy. By combining well-known antibiotics with an innovative adjuvant, Calygen delivers:
- A triple-action regimen to tackle resistant Gram-negatives.
- A carbapenem-sparing strategy: proven efficacy vs MDR UTI and other severe infections, preserving carbapenems for last-resort use.
- A well-characterized safety profile, facilitating prescribing confidence.
- Support for antimicrobial stewardship: reduces reliance on broad-spectrum carbapenems and polymyxins.
CALOHIE CG’s robust clinical data and unique mechanism make it an attractive option for infectious disease specialists and hospitalists managing ESBL/MBL Gram-negative infections.
Sources: CALOHIE CG’s profile and studies.


